After Chemical Weathering, What Is True About The Chemical Makeup Of The Weathered Rock?
Affiliate 5 Weathering and Soil
5.2 Chemical Weathering
Chemical weathering results from chemical changes to minerals that get unstable when they are exposed to surface conditions. The kinds of changes that take place are highly specific to the mineral and the environmental conditions. Some minerals, like quartz, are near unaffected past chemical weathering, while others, like feldspar, are hands altered. In full general, the degree of chemical weathering is greatest in warm and wet climates, and least in cold and dry climates. The important characteristics of surface conditions that lead to chemical weathering are the presence of water (in the air and on the footing surface), the abundance of oxygen, and the presence of carbon dioxide, which produces weak carbonic acid when combined with h2o. That process, which is key to most chemical weathering, can be shown as follows:
H2O + COtwo —->H2CO3 then HtwoCO3 —-> H+ + HCOiii –,
water + carbon dioxide —-> carbonic acrid then carbonic acid —-> hydrgen ion + carbonate ion
Here we take water (e.k., every bit rain) plus carbon dioxide in the atmosphere, combining to create carbonic acid. Then carbonic acid dissociates (comes apart) to form hydrogen and carbonate ions. The amount of CO2 in the air is enough to make only very weak carbonic acid, just there is typically much more CO2 in the soil, and so water that percolates through the soil can become significantly more than acidic.
There are ii main types of chemical weathering. On the one manus, some minerals become altered to other minerals. For example, feldspar is altered — by hydrolysis — to clay minerals. On the other hand, some minerals dissolve completely, and their components go into solution. For example, calcite (CaCO3) is soluble in acidic solutions.
The hydrolysis of feldspar tin be written like this:
CaAl2Si2O8 + H2CO3 + ½O2 —-> AltwoSi2O5(OH)4 + Ca2+ + COthree two-
plagioclase + carbonic acrid —-> kaolinite + dissolved calcium + carbonate ions
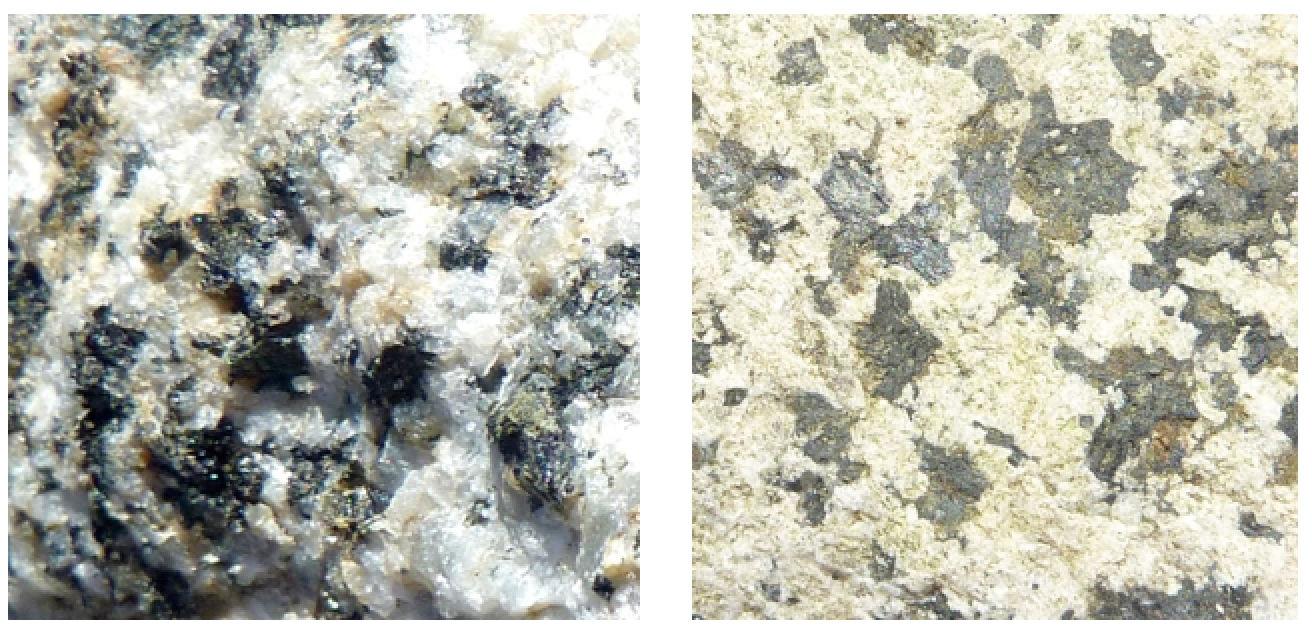
This reaction shows calcium plagioclase feldspar, but similar reactions could also be written for sodium or potassium feldspars. In this case, we terminate up with the mineral kaolinite, along with calcium and carbonate ions in solution. Those ions tin can eventually combine (probably in the sea) to form the mineral calcite. The hydrolysis of feldspar to dirt is illustrated in Figure 5.ix, which shows two images of the same granitic rock, a recently broken fresh surface on the left and a clay-contradistinct weathered surface on the right. Other silicate minerals tin besides get through hydrolysis, although the stop results will be a little different. For example, pyroxene tin can exist converted to the clay minerals chlorite or smectite, and olivine can be converted to the clay mineral serpentine.
Oxidation is another very important chemical weathering process. The oxidation of the atomic number 26 in a ferromagnesian silicate starts with the dissolution of the iron. For olivine, the process looks like this, where olivine in the presence of carbonic acid is converted to dissolved iron, carbonate, and silicic acid:
FetwoSiO4+ 4HtwoCO3—> 2Fe two+ + 4HCOthree – + H4SiO4
olivine + (carbonic acid) —> dissolved iron + dissolved carbonate + dissolved silicic acrid
In the presence of oxygen, the dissolved atomic number 26 is and then quickly converted to hematite:
2Fe 2+ + 4HCO3– + ½ O2 + 2HiiO —->Atomic number 262Othree + 4H2COthree
dissolved atomic number 26 + bicarbonate + oxygen + water—->hematite + carbonic acid
The equation shown here is for olivine, but it could utilise to almost any other ferromagnesian silicate, including pyroxene, amphibole, or biotite. Iron in the sulphide minerals (eastward.yard., pyrite) can also be oxidized in this way. And the mineral hematite is not the just possible finish result, as there is a wide range of iron oxide minerals that tin can form in this way. The results of this process are illustrated in Figure five.10, which shows a granitic rock in which some of the biotite and amphibole have been altered to class the iron oxide mineral limonite.

A special type of oxidation takes place in areas where the rocks have elevated levels of sulphide minerals, especially pyrite (FeStwo). Pyrite reacts with water and oxygen to form sulphuric acid, as follows:
2FeS2+ 7O2 +2HiiO —–> 2Fetwo+ HiiThen4+ 2H+
pyrite + oxygen + water —–> iron ions + sulphuric acid + hydrogen ions
The runoff from areas where this procedure is taking identify is known every bit acid rock drainage (ARD), and even a rock with 1% or ii% pyrite tin can produce significant ARD. Some of the worst examples of ARD are at metal mine sites, especially where pyrite-bearing rock and waste product material have been mined from deep hole-and-corner and then piled up and left exposed to h2o and oxygen. One case of that is the Mt. Washington Mine near Courtenay on Vancouver Island (Figure 5.11), but there are many like sites across Canada and around the globe.

At many ARD sites, the pH of the runoff water is less than 4 (very acidic). Under these conditions, metals such as copper, zinc, and lead are quite soluble, which tin can lead to toxicity for aquatic and other organisms. For many years, the river downstream from the Mt. Washington Mine had so much dissolved copper in it that it was toxic to salmon. Remediation work has since been carried out at the mine and the state of affairs has improved.
The hydrolysis of feldspar and other silicate minerals and the oxidation of iron in ferromagnesian silicates all serve to create rocks that are softer and weaker than they were to begin with, and thus more susceptible to mechanical weathering.
The weathering reactions that we've discussed so far involved the transformation of one mineral to some other mineral (e.chiliad., feldspar to clay), and the release of some ions in solution (e.1000., Ca2+). Some weathering processes involve the complete dissolution of a mineral. Calcite, for example, volition deliquesce in weak acid, to produce calcium and bicarbonate ions. The equation is every bit follows:
CaCO3 + H+ + HCOiii – —–> Catwo+ + 2HCOiii –
calcite + hydrogen ions + bicarbonate —–> calcium ions + bicarbonate
Calcite is the major component of limestone (typically more than 95%), and under surface conditions, limestone will dissolve to varying degrees (depending on which minerals it contains, other than calcite), as shown in Figure v.12. Limestone also dissolves at relatively shallow depths underground, forming limestone caves. This is discussed in more than detail in Chapter 14, where we look at groundwater.

Practice v.2 Chemical Weathering
The main processes of chemical weathering are hydrolysis, oxidation, and dissolution. Complete the following table by indicating which process is primarily responsible for each of the described chemical weathering changes:
| Chemical Change | Procedure? |
|---|---|
| Pyrite to hematite | |
| Calcite to calcium and bicarbonate ions | |
| Feldspar to clay | |
| Olivine to serpentine | |
| Pyroxene to iron oxide |
After Chemical Weathering, What Is True About The Chemical Makeup Of The Weathered Rock?,
Source: https://opentextbc.ca/geology/chapter/5-2-chemical-weathering/
Posted by: compoorwastincer.blogspot.com


0 Response to "After Chemical Weathering, What Is True About The Chemical Makeup Of The Weathered Rock?"
Post a Comment